Current and Past team members
PRISMS collaborators for Mg corrosion include David Montiel, Katsuyo Thorton & Steve Dewitt (UM)
Our contribution
Role of microstructure on corrosion rates and mechanisms of Mg alloys
This work is part of our participation in the DOE-BES funded Center for PRedictive Integrated Structural Materials Science (PRISMS).
Mg, being a light material, presents great potential as a structural material for transportation applications provided that strength, ductility, fracture, and corrosion behavior can be controlled. Engineering solutions such as coatings have enabled the use of Mg alloys, despite their intrinsic low corrosion resistance. Yet, the fundamental mechanisms responsible for the unique localized corrosion behavior of bare Mg alloys, the associated abnormal hydrogen evolution response, and the relationships between corrosion behavior and alloy microstructure are still unsolved and bare important consequences for not only structural use but also battery applications.
Pen-Wei’s thesis investigated the specificities of Mg corrosion and the roles of alloy chemistry and microstructure that had been overlooked until now. Multiscale site-specific microstructure characterization techniques, including in situ optical microscopy, scanning electron microscopy with focused ion beam milling, and transmission electron microscopy, combined with electrochemical analysis and hydrogen evolution rate monitoring, were performed on pure Mg and selected Mg alloys under free corrosion and anodic polarization, revealing key new information on the propagation mode of localized corrosion and the role of alloy microstructures, thereby confirming or disproving the validity of previously proposed corrosion models.
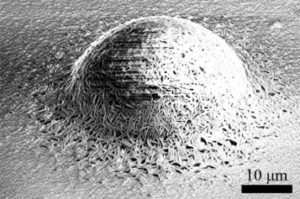
Uniform surface corrosion film on Mg alloys immersed in NaCl solution consisted a bilayered structure, with a porous Mg(OH)2 outer layer on top of a MgO inner layer. Presence of fine scale precipitates in Mg alloys interacted with the corrosion reaction front, reducing the corrosion rate and surface corrosion film thickness. Protruding hemispherical dome-like corrosion products, accompanied by growing hydrogen bubbles, formed on top of the impurity particles in Mg alloys by deposition of Mg(OH)2 via a microgalvanic effect. Localized corrosion on Mg alloys under both free immersion and anodic polarization was found to be governed by a common mechanism, with the corrosion front propagating laterally a few μm inside the alloy and underneath the surface corrosion film, with finger-like features aligned with (0001) Mg basal planes at the localized corrosion/alloy interface. Rising streams of hydrogen bubbles were found to follow the anodic dissolution of Mg and formation of Mg(OH)2 corrosion products at the propagating localized corrosion fronts. Alloying elements segregation to the grain boundaries showed the ability to stop localized corrosion propagation momentarily.
By revealing the microstructure of corrosion features on Mg alloys, qualitative relationships between the corrosion behavior and alloy microstructures were identified. This microscopic information can serve as a guideline for future development of Mg alloys by tailoring the microstructure to achieve proper corrosion responses for applications under different environments, which is the object of our current investigations.
In situ optical microscopy of the corrosion process taking place on a Mg alloy
Role of subsurface deformation on the corrosion mechanisms of 304 stainless steel
The oxide products found on austenitic stainless steels after high temperature exposures in nuclear reactor environments are well documented, but the mechanisms for the oxide formation are still ambiguous. One issue of practical importance is the role of surface deformation on the oxidation response. To address this question, short high-temperature water exposures were conducted on electropolished and ground surfaces of Type 304 stainless steel. Characterization of the developing oxide scales using transmission electron microscopy and atom probe tomography revealed significantly different responses from the two surface finishes. In the absence of sub-surface deformation, the oxide scale grows as an equilibrium phase of uniform composition with accumulation of Cu to the metal/oxide interface. In the presence of significant sub-surface deformation, a non-equilibrium nanostructured oxide scale develops as a result of selective oxidation along fast diffusion paths.
Facilities
Data
Through this project, we also support the development of Materials Commons. Some of our data can be accessed:
Publications
- Linking the Microstructure of a Heat-Treated WE43 Mg Alloy with its Corrosion Behavior, P-W Chu, EA Marquis, Corrosion Science 101 94-104 link
- Anodic Hydrogen Evolution and Localized Corrosion during Galvanostatic Polarization of a Peak-Aged Mg-Y-Nd-Zr Alloy, P-W Chu, EA Marquis, Journal of The Electrochemical Society 163 (8), C402-C409 (2016) link
- Anodic Hydrogen Evolution and Localized Corrosion during Galvanostatic Polarization of a Peak-Aged Mg-Y-Nd-Zr Alloy, P-W Chu, EA Marquis, Journal of The Electrochemical Society 163 (8), C402-C409 (2016) link
- Sensitization and Stress Corrosion Crack Response of Dual Certified Type 304/304L Stainless Steel, KB Fisher, BD Miller, EC Johns, R Hermer, C Brown, EA Marquis, Corrosion (2018) link
- The role of surface deformation in the oxidation response of Type 304 SS in high temperature deaerated water, KB Fisher, BD Miller, EC Johns, EA Marquis, Corrosion Science (2018) link
Selected Presentations
- P.W. Chu, “Negative Difference Effect and Localized Corrosion on a T6-WE43 Mg Alloy in Unbuffered and Buffered NaCl Solutions”, Presentation at Corrosion 2016, March 6-10, 2016. Vancouver, BC, Canada.
- P.W. Chu, Linking WE43 Mg alloys Microstructure to Local Corrosion Behavior in NaCl Solutions, Corrosion 2015, March 15-19, 2015, Dallas TX.
- P.W. Chu, Presentation at the Gordon Research Conference on Aqueous Corrosion, July 2014. Colby Sawyer College, NH.
U.S. Department of Energy – Basic Energy science program [DE-SC0008637] as part of the Center for PRedictive Integrated Structural Materials Science (PRISMS Center)


